MUMBAI: Hyderabad-based Natco Pharma has sued Danish drugmaker Novo Nordisk, maker of blockbuster weight-loss drug Wegovy (semaglutide), claiming its version of the drug does not infringe upon the device or process patent technology owned by the multinational company. The case came up for hearing in Delhi High Court on Wednesday.
"The court has directed the parties to engage in a 'pre-litigation mediation', an attempt to bury the dispute through mutual settlement before the case can be heard on deeper nuances such as non-infringement of patents," a senior lawyer well-versed with the case told ET on condition of anonymity.
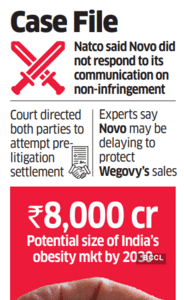
The lawyers representing Natco Pharma informed the court that over the past few months it had approached Novo Nordisk to communicate on its non-infringing patents but received no response, said people familiar with the matter.
"A clear communication from the innovator may trigger a generic launch of the drug in India, sinking the prospects for Wegovy's sales and so that may be seen as a delaying tactic," said an expert, who did not wish to be identified.
Novo Nordisk launched Wegovy in India in June, trailing its global rival Eli Lilly, which launched its patented brand Mounjaro (tirzepatide) in March. Recently, Mounjaro was launched in the easy-to-use pen device form, an improvement over its earlier versions of injectable syringes.
Natco Pharma did not comment on the development, while Novo Nordisk did not respond to queries sent by ET.
The development comes months after Novo Nordisk in May sued Dr Reddy's Labs and OneSource, a contract manufacturing company, in Delhi High Court alleging infringement of its valid patents. The Danish company also claimed that the two companies were importing large quantities of the semaglutide raw material to make and export the formulations.
However, the court called upon Novo Nordisk to submit the evidence since there was no substantive proof to back such claims. Earlier that month, Dr Reddy's Labs had filed a lawsuit for revocation of Novo Nordisk's patents.
The patent for semaglutide in India is set to expire in March next year, potentially opening the floodgates for at least seven companies to launch their versions. This is expected to result in the prices plummeting at least 80% from the present levels of '17,000-26,000 per month.
The market for obesity drugs in India is expected to rapidly expand to '8,000 crore by 2030 from '700 crore at present, fuelled by aggressive marketing for such drugs and a faster adoption by doctors and patients.
"The court has directed the parties to engage in a 'pre-litigation mediation', an attempt to bury the dispute through mutual settlement before the case can be heard on deeper nuances such as non-infringement of patents," a senior lawyer well-versed with the case told ET on condition of anonymity.
The lawyers representing Natco Pharma informed the court that over the past few months it had approached Novo Nordisk to communicate on its non-infringing patents but received no response, said people familiar with the matter.
"A clear communication from the innovator may trigger a generic launch of the drug in India, sinking the prospects for Wegovy's sales and so that may be seen as a delaying tactic," said an expert, who did not wish to be identified.
Novo Nordisk launched Wegovy in India in June, trailing its global rival Eli Lilly, which launched its patented brand Mounjaro (tirzepatide) in March. Recently, Mounjaro was launched in the easy-to-use pen device form, an improvement over its earlier versions of injectable syringes.
Natco Pharma did not comment on the development, while Novo Nordisk did not respond to queries sent by ET.
The development comes months after Novo Nordisk in May sued Dr Reddy's Labs and OneSource, a contract manufacturing company, in Delhi High Court alleging infringement of its valid patents. The Danish company also claimed that the two companies were importing large quantities of the semaglutide raw material to make and export the formulations.
However, the court called upon Novo Nordisk to submit the evidence since there was no substantive proof to back such claims. Earlier that month, Dr Reddy's Labs had filed a lawsuit for revocation of Novo Nordisk's patents.
The patent for semaglutide in India is set to expire in March next year, potentially opening the floodgates for at least seven companies to launch their versions. This is expected to result in the prices plummeting at least 80% from the present levels of '17,000-26,000 per month.
The market for obesity drugs in India is expected to rapidly expand to '8,000 crore by 2030 from '700 crore at present, fuelled by aggressive marketing for such drugs and a faster adoption by doctors and patients.
You may also like

'Terrorists emerged out of several tunnel shafts': Video shows Israel repel major Hamas assault on its forces; watch

California plague case: Lake Tahoe resident bitten by infected flea, contracts plague; patient under medical supervision

'Try to fix the Republican Party': JD Vance asks Musk to stand by GoP; amid buzz on Tesla CEO's new political outfit

US Navy warship blaze: Two sailors injured as USS New Orleans burns for 12 hours near Okinawa; Japan aids firefight

Madras Day at Sheraton Grand Chennai Resort & Spa, Mahabalipuram







